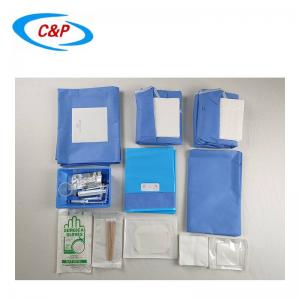
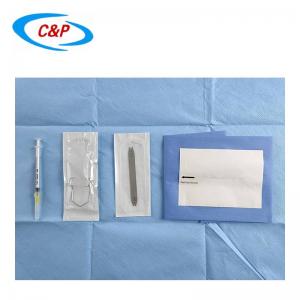
Surgical fenestrated drape is a surgical drape used during surgical procedures to create a sterile barrier between the patient's body and the surgical site. It is designed to provide a clear and accessible area, known as the fenestration or surgical opening, where the surgical procedure will be performed.
The size and shape of the fenestration will depend on the specific surgical procedure. Some fenestrations may have adhesive edges or flaps to secure the drape around the surgical site.
PRODUCT PARAMETERS
|
Name |
Fenestrated Drape |
|
Material |
SMS, PP, PE, Spunlace Nonwoven |
|
Thickness |
20 -100gsm |
|
Color |
Blue or as request |
|
Size |
100x75cm, fenestration diameter 6cm or as your request |
|
Packing |
1pc/sterile pack, 300pcs/ctn, ctn size:60x40x40cm or as request |
|
Shipping Port |
Shanghai, Ningbo |
|
OEM |
Material, Size, LOGO, Package or other specifications |
|
Quality Certification |
CE, ISO13485, EN13795 |
|
Sterile |
EO Sterile |
PRODUCT DISPLAY

FEATURES
1. Sterility: Surgical drapes are sterile, meaning they are free from microorganisms that can cause infection during surgery.
2. Waterproof: Surgical drapes are waterproof, meaning they can withstand contact with bodily fluids, blood, and other fluids that may be present during surgery. This feature helps to maintain a clean and dry surgical field.
3. Non-irritating: Surgical drapes are made of materials that are non-irritating to the skin, reducing the risk of allergic reactions or skin irritation during surgery.
4. Breathable: Surgical drapes are designed to allow air to pass through them, reducing the risk of heat buildup and maintaining a comfortable working environment for the surgeon and surgical team.
5. Easy to handle: Surgical drapes are designed to be easy to handle and manipulate during surgery, allowing the surgeon to work efficiently and effectively.
6. Durable: Surgical drapes are made of high-quality materials that are durable and long-lasting, ensuring that they can withstand use and sterilization processes without losing their effectiveness.
COMPANY PROFILE

Our company was founded in 2007, committed to the research and development, production and sales of medical consumables industry, covering an area of 5000 square meters, with more than 100 employees. All products produced by our factory have passed EU CE certification. In terms of quality inspection, we have established corresponding quality control procedures in strict accordance with the requirements of ISO13485:2016 quality system and EN13795 standard to ensure the standardized production and quality control of products. In the market, the products are sold to South Africa, Southeast Asia and Europe. Our after-sales service and technical support to customers have also been recognized by customers.
Our main surgical packs are Universal pack, C-section pack, Baby Delivery Pack, Angiography Pack, Ophthalmic Pack, Extremity pack, Arthroscopy pack, Hip pack, Dental pack, Cystoscopy pack, TUR pack, Cardiovascular pack, ENT pack, etc.
OUR ADVANTAGES
1. Rich production experience: With 17 years of experience in the production of medical consumables, valuable knowledge and expertise have been accumulated in this field. Enable us to better understand the needs and requirements of the industry, in order to produce high-quality products.
2. Compliance with international standards: CE, ISO13485 certificate is an internationally recognized standard for quality management in the medical device industry. This certification demonstrates that our products and processes comply with strict safety and efficacy regulatory requirements, ensuring that our product is suitable for its intended use.
3. ISO 8 clean room: ISO 8 clean rooms provide a highly controlled environment with limited particulate matter in the air. This cleanliness is crucial for the production of certain medical consumables, as it can reduce the risk of product contamination and ensure sterility.
4. Good performance: Exported to more than 20 countries.
5. Design team: We have a professional design team can do OEM&ODM.
OUR CERTIFICATE

OUR EXHIBITION

RECOMMENDED PRODUCTS