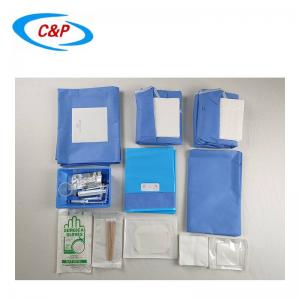
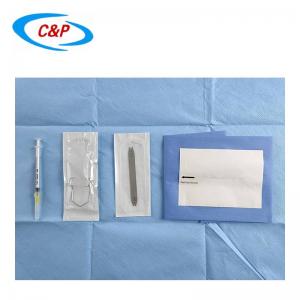
SMS Disposable Sterile Shoulder Arthroscopy Surgical Drape
Product Description:
SMS nonwoven shoulder arthroscopy drape is made of SMS,SMMS and PE film + nonwoven fabric , the medical shoulder arthroscopy drape has strict quality inspection , which can protects patients and staff from bacterial infection during surgery.
Details are as follows:
1). Sterile: EO
2). Customized size and hole size is available
3). Material: SMS, SMMS, PE film + non woven fabric
4). Color: Blue
5). Certificate: CE, ISO13485, EN13795
6). Package: 1pc/sterile pack, 20pcs/ctn
7). Specification:
|
REF |
Size |
Description |
|
21202 |
250x160 |
Split 15x50cm, Fluid collection pouch 115x27cm with 2 alve, Tape 5cm |
Pictures:


Product Features:
1). Ultra-thin and comfortable film makes it stick to surgical incision and surgical towels which will contribute to operation.
2). Its features of transparent and breathable allows the skin breathe freely, prevents the water vapor under the towel from gathering, reduces possibility of infection. That can provide a sterile environment for the operation.
3). The common bacteria, such as enteropathogenic bacterial, pyococcus, pathogenic yeast, can be killed.
Our advantages:
1). 13 years experience, surgical drapes, packs, surgical gown, masks, isolation gowns, equipment covers and other disposable nonwoven medical products.
2). We set up 100000 grade standard clean room(ISO8) space.
3). Excellent packaging and warehousing facilities.
4). Custom Designed Drapes & Packs, OEM supplier.
5). Flexible base material options for drape.
6). Timely execution of orders.
7). Competitive pricing.
Our company:

Founded in 2007, our company is committed to the research and development, production and sales of medical consumables industry, covering an area of 3,000 square meters, more than 100 employees. All products produced by the factory have passed the CE certification of the European Union and the ethical, safety and production certification of many international enterprises. In terms of quality inspection, we have established corresponding quality control procedures in strict accordance with the requirements of ISO13485:2016 quality system to ensure the standardized production and quality control of products. In terms of product innovation, we ensure the leading products/standard to industry-leading products through financial incentives. In the market, products are sold to South Africa, southeast Asia, Europe. Our after-sales service and technical support for customers have also been recognized by customers.